Genetic testing is now available for many disorders. We have known for centuries that heredity is involved in many diseases. However, 15 years ago the Human Genome project was completed, and the sequence of human DNA is now known. This major accomplishment has caused an explosion of new information about genetic diseases, confirming previous suspicions in many diseases and identifying the genetic contribution in others.
A dramatic increase in genetic tests has become available not only to researchers and clinicians but also to any interested individual through direct-to-consumer vendors. The cost of this testing has fallen dramatically. When done properly, genetic testing has been
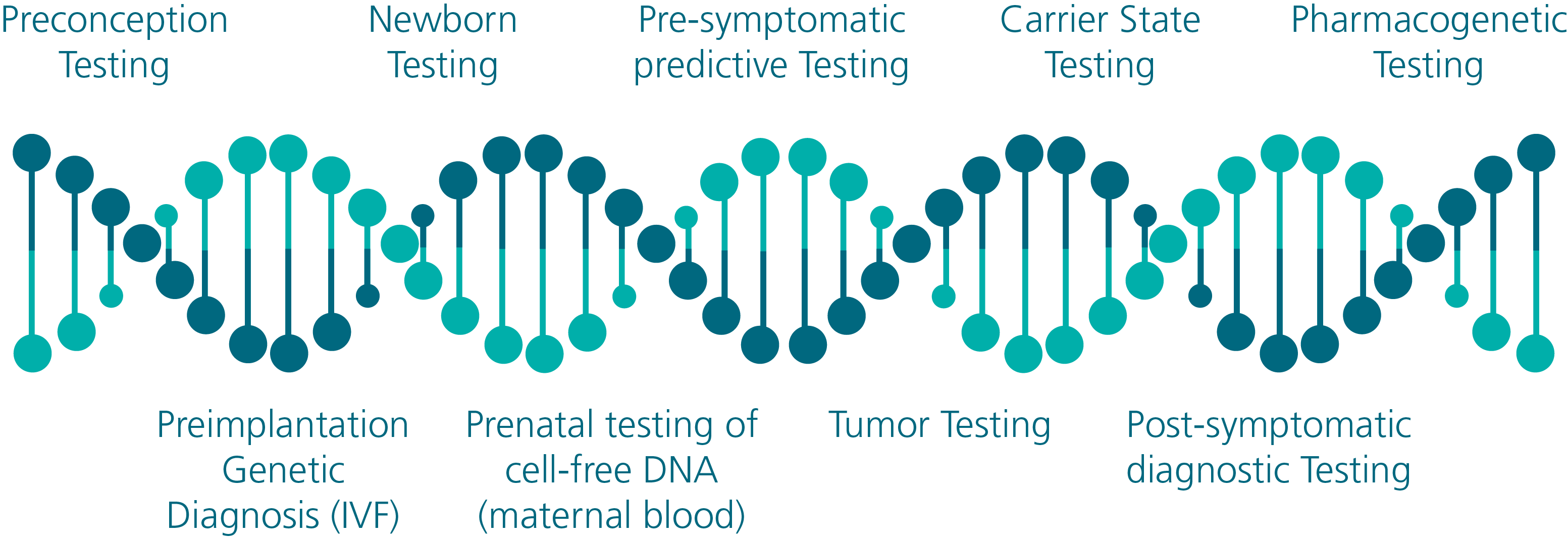
shown to be accurate and reproducible. A list of genetic testing opportunities that are now available is displayed in Figure 1.
Figure 1 - Genetic Testing Opportunities
As often occurs with new technology or scientific advances, there is a delay in gathering enough population data to make reliable judgements about long term outcomes. Scientists and physicians rely on evidence-based data, and with any new technology there is a delay in obtaining this information.
Long term studies need, well, long terms. Therefore, in 2018, patients don’t always know which genetic diseases are present in their family, which diseases have tests that would be helpful or which medical professionals to consult to have the testing performed or the results explained.
Fortunately, there are many ongoing studies to gather information on how best to use genetic testing in individual diseases. In addition, population management studies are being performed to evaluate the impact of large scale genetic testing on the general
population.
More clinical research is needed to identify which tests have the greatest impact on mortality. Questions on whom should be tested, when they should be completed and which tests to perform have been only partially answered.
Improving Mortality Risk?
As genetic testing has become more available, clinicians now are able to combine genetic as well as non-genetic information to predict the development and/or severity of many diseases. As these genetic risks are being identified, one big question has been at the forefront: Is genetic testing improving mortality risk?
Mortality improvement would be expected only if an outcome from modifying behavior is available and the individual is willing to modify the behavior. Examples of behavior change could include more frequent monitoring/screening, modification of lifestyle risks
and/or improved medication adherence. In some cases, disease modifying surgeries such as mastectomies, oophorectomies or colectomies have been shown to modify risk.
Currently, not all genetic diseases have effective disease modifying behavior changes available to significantly reduce risk (e.g., Huntington’s’ disease). However, there is a growing list of diseases where specific behavior changes have been shown to reduce mortality risk.
Many inherited diseases have intervention options that can favorably impact mortality. Examples of diseases in which specific behavior changes have been shown to significantly improve risk include those in Figure 2.
Figure 2 - Specific Diseases and Behavior Changes Associated with Mortality Improvement
| Lynch Syndrome | - Frequent screening for cancer |
| BRCA Mutation | - Frequent screening for cancer
- Disease modifying surgery
(mastectomy/salpingo-oopherectomy)
- Hormonal treatment |
| Familial Adenomatous Polyposis | - Frequent screening for cancer
- Disease modifying surgery (coloectomy) |
| Hemochromatosis | - Frequent phlebotomies |
| Cystic fibrosis | - CFTR modulator treatment
- Vaccination
- Prompt infection treatment |
| PKU | - Dietary restriction
- Pharmacotherapy |
Cytochrome P450 2D6 ultra-fast or
poor metabolizer characteristics
(pharmacogenetic testing) | - Modification of medication dosage or
selection |
| Tumor Genetic Testing | - Chemotherapy selection modification |
To illustrate one of the above examples, let’s review pharmacogenetic testing. Pharmacogenetic testing has the potential to dramatically impact a person’s use of medications.
Based upon the individual’s medication metabolism characteristics, one can avoid medications that are ineffective or cause serious side effects. In other cases, the dosage can be modified based upon the individual’s personal metabolism characteristics to
maximize benefit and avoid complications.
Figure 3 - Cytochrome P450 2D6 Pharmacogentic Testing
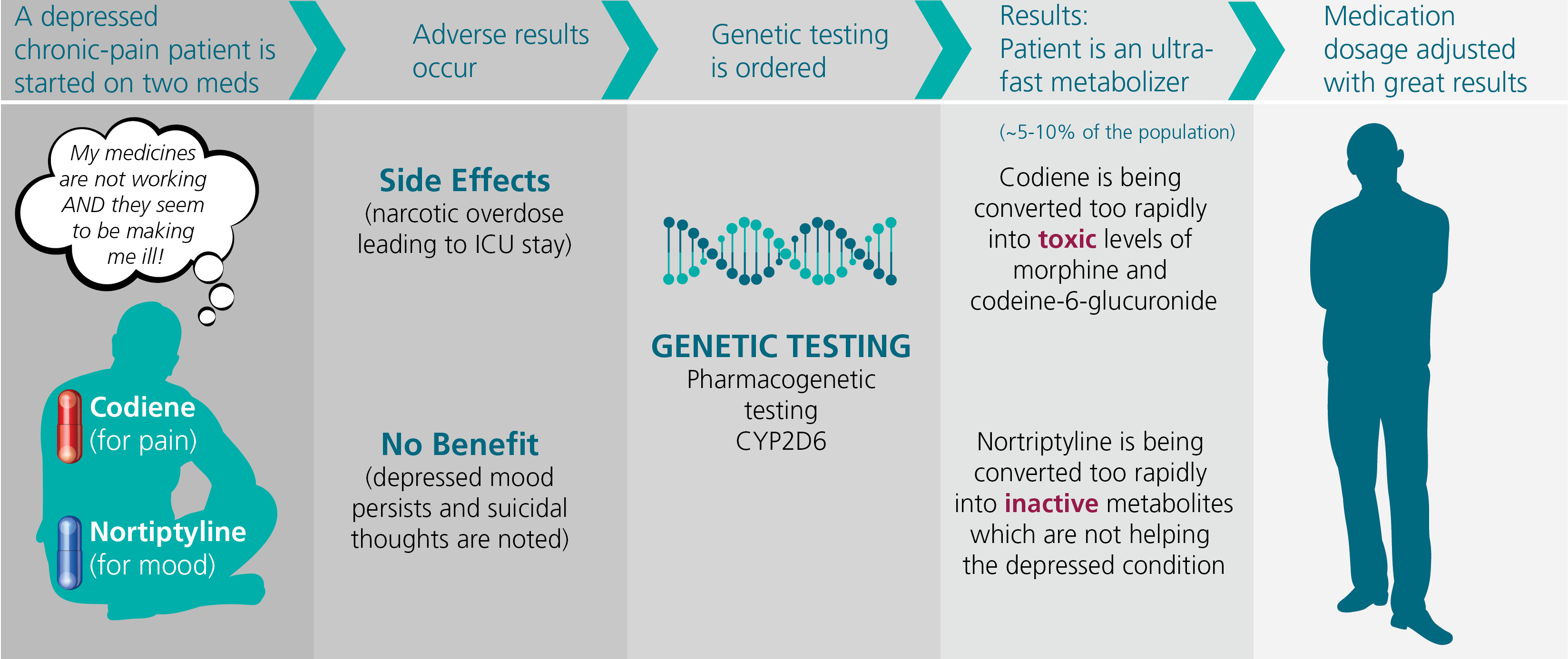
Click on image to display in larger size
Figure 3 illustrates the potential benefit of cytochrome P450 2D6 pharmacogenetic metabolism testing. Approximately 25% of medications are metabolized by this pathway, including such common medications as some antidepressants and pain medications.
While 80% of people metabolize these medications at a predictable rate, approximately 5-10% of the population are ultra-fast metabolizers and another 5-10% of people are poor
metabolizers.
Some medications such as codeine are prodrugs and require metabolism in the body to begin working. Other medications such as nortriptyline, which is used for depression, work immediately when introduced into the system but are converted into inactive
metabolites by this pathway. Thus, an ultra-fast metabolizer can experience a narcotic overdose with typical dosages of codeine.
But when given nortriptyline, the medication is quickly converted into inactive metabolites, hampering its effectiveness. Knowing the speed of metabolism can influence the dosage of these medications, making them safer and more beneficial.
The Goal of Predictive Testing
Identifying the genetic disease prior to the onset of symptoms or adverse results is the goal of pre-symptomatic predictive testing. Newborn screening is an example and has been occurring since the 1960s.
In the US and Canada 98% of all newborns undergo testing. The number of genetically linked disorders tested for at birth in the US varies state by state; however, most newborns are screened for at least 29 conditions. These include testing for hemoglobinopathies such as sickle cell anemia (1:5,000), amino acid disorders like
phenylketonuria (1:20,000) or other disorders such as cystic fibrosis (1:5,000). When found, treatments can be started immediately which leads to lessened morbidity and improved mortality outcomes.
Cystic fibrosis, for instance, is diagnosed at 0.5 months when newborn screening is done, 0.2 months if meconium ileus occurs in the infant and 14.5 months in all others. The CDC reports that the diagnosis based on symptoms creates a greater than two-fold increase in medical complications over diagnosis resulting from screening. Observational studies have shown a consistently lower mortality rate among screened compared to unscreened infants.
Lynch Syndrome
Pre-symptomatic testing can involve testing young adults with increased risk. Lynch syndrome is a genetic condition involving a mutation in one of several genes which produce mismatch repair proteins. Those afflicted have an increased risk of developing
cancer in their lifetime.
Colon, endometrium, stomach, ovarian and urinary tract cancers are just a few of the cancers associated with this condition. The lifetime chance of developing cancer is estimated to be up to 80% of those with the genetic mutation. Afflicting approximately one
out of every 370 people, it is estimated that only 1.2% of people know they have it.
In Lynch syndrome cancer can be prevented or detected early enough to significantly impact mortality. In a well-done 2013 study, H. Jarvinen et. al. documented that colorectal cancer screening can decrease the incidence of invasive colon cancer by 62%. Mortality
was estimated to be decreased by 65%. Compliance with the frequent colonoscopy screening suggested in one study was 80%.
Even when the recommendation was much more difficult, such as undergoing disease modifying surgery (hysterectomy and bilateral salpingo-oopherectomy to prevent pelvic cancer), 19% proceeded with the recommendation.
BRCA mutation
Another example of pre-symptomatic, predictive testing is testing for the BRCA mutation in those at increased risk. While the incidence of having a deleterious BRCA mutation is approximately 1:400, the lifetime risk of cancer when the mutation is present can
be high.
BRCA mutations are associated with an increased risk of cancer in several different sites for men and women but the highest risk is for breast cancer in women (55-70% risk in carriers to age 70 years). The advantage of early identification of this increased risk
is the ability to be educated and participate in proven effective treatments in reducing mortality risk.
These treatments include frequent breast imaging (MRI and mammography), chemoprevention and risk-reducing surgery. Chemoprevention includes taking medications such as Tamoxifen (50% reduction in invasive breast cancer when taken for five years).
Risk-reducing surgery, for instance, includes bilateral prophylactic mastectomy (95% reduction in breast cancer) or bilateral prophylactic salpingo-oopherectomy (90% reduction in ovarian cancer, 50% reduction in breast cancer). The rate of women
undergoing contralateral prophylactic mastectomy with breast cancer has been estimated to be 50%.
Similar results (53%) were found in one study for bilateral prophylactic salpingo-oopherectomy and/or bilateral prophylactic mastectomy in women identified as being high risk and with a known BRCA mutation but with no personal history of cancer.
Avoiding the disease (along with its mortality implications) is the rationale behind carrier testing. Prospective parents can be tested for many of the autosomal recessive diseases to determine the risk of their children inheriting the disease.
Carrier couples have several options. These options include choosing not to have any further biological children, accepting the 25% risk with each subsequent child, having a child by heterologous fertilization or having in vitro fertilization and a preimplantation
genetic evaluation. Several studies have shown a reduction in the incidence of some diseases based upon carrier screening.
Adherence to Recommended Interventions is not 100%
Despite examples of helpful behavior change options after identification of genetic risk, there are hurdles people face in implementing these changes. Similar struggles are encountered when high risk individuals are identified using nongenetic testing.
For example, in well-designed studies, individuals who are given a diagnosis of hypertension or hyperlipidemia based upon nongenetic risk information who are asked to modify their diet, activity level and/or regularly take medication have a difficult time
adhering to these recommendations long term.
Figure 4 - Adherence to Recommended Interventions
| Hyperlipidemia | Statin medication | 10 years after diagnosis:
- Meds taken 42% of days |
| Hypertension | Antihypertensive medication | 6 months after diagnosis:
- 8.1% high adherence
- 40.5% intermeidate adherence
- 51.4% low adherence |
Prostrate Cancer
Screening | PSA testing (2010 data prior to USPSTF change in recommendation) | Annual testing:
- 37.8% of men >50 y/o |
Breast Cancer Screening
in Women of Average
Risk | Mammogram
(2015 data) | X-ray within the past 2 years:
- 65.3% of women >40 y/ |
Likewise, behavior change regarding lifestyle proves to be very difficult for most people. Obese individuals frequently struggle to lose weight; alcoholics struggle with discontinuing alcohol; and smokers struggle with quitting cigarettes. Genetic testing and identifying a strong genetic component to the risk of these adverse behaviors has been shown to have little additional benefit.
Armed with genetic information, factors that could play a role in whether an individual significantly changes behavior might include:
- Presence and severity of symptoms
- Mortality risk
- Penetrance of the disease (how likely when identified as having genetic predisposition that the disease occurs)
- Typical age of onset
- Education level
- Cost (psychological, time and financial) of the suggested behavior change
- Side effects of the suggested behavior change
- Fully understanding the disease, the genetic testing results and the suggested behavior changes
- An adequate support system which might include encouragement, education and coaching from family members and professional staff
- Difficulty of implementing the change
In summary, accurate genetic testing is now available for many but not all inherited diseases. Based upon this information in many but not all diseases, there are effective treatments available to alter mortality risk. Many people, but not all, when appropriately tested and counseled, modify their behaviour to reduce the risk.
Clearly, there is a huge opportunity for genetic testing to have a significant beneficial impact not only in one individual’s life but also in the entire population. However, more studies are needed to further define how best to implement genetic testing in clinical
medicine.
References
- Benner JS, Et al Long-term persistence in use of statin therapy in elderly patients. JAMA 288:455-461
- Castellani, Carlo, et al. Association Between Carrier Screening and Incidence of Cystic Fibrosis. JAMA. 2009;302 (23):2573-2579
- Grosse Scott, et al. Newborn Screening for Cystic Fibrosis. Evaluation of Benefits and Risks and Recommendations for State Newborn Screening Programs. MMWR. CDC. October 14, 2004.
- Guillen, C et al DOI: 10.1200/JCO2016.34.15 suppl.e13054. Journal of Clinical Oncology 34, no. 15
- Hollands, Gareth, et al. The impact of communicating genetic risks of disease on risk-reducing health behavior: systematic review with meta-analysis. BMJ 016;352: 1102
- Jarvinen, HJ, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology. 2000;118(5):829
- Jemal A et al. Prostate Cancer Incidence and PSA Testing patterns in Relation to USPSTF Screening Recommendations. JAMA. 2015;314(19): 2054-2061
- Mazzaglia G, et al Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 120:1598-1605
- National Cancer Institute website. Cancer.gov https://www.cancer.gov/types/breast/risk-reducing-surgery-fact-sheet
Last accessed 6/28/2018. - Palomaki, Glenn et al EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genetics in Medicine. Volume 11, Jan, 2009
- Schneider Kai and Schmidtke, Jorg. Patient compliance based on genetic medicine: a literature review. J Community Genetics (2014) 5:31-8
- Stuckey, A. et al. (2010). Clinical characteristics and choices regarding risk-reducing surgery in BRCA mutation carriers. Gynecologic and Obstetric Investigation. 69(4), 270-273.
- Tinley, S, Lynch, H et al 2004 Feb 15, American Journal of Medical Genetics 125A
- Tollin, S. Prophylactic, Risk-Reducing Surgery in Unaffected BRCA-Positive Women: Quality of Life, Sexual Functioning and Psychological Well-Being. Graduate Theses and Dissertation. (2011).
- US Department of Health and Human Services. Health, United States, 2016. DHHS Publication No. 2017-1232